On June 8, 2020, Dr, Jie Na’s group published a research paper entitled "Homozygous MESP1 knock-in reporter hESCs facilitated cardiovascular cell differentiation and myocardial infarction repair" in Theranostics, IF = 8.06 (the year 2018). Dr. Jie Na’s team constructed a human cardiovascular progenitor reporter cell line MESP1-mTomato, which facilitated the differentiation of cardiomyocytes, vascular endothelial cells, and smooth muscle cells. It could also potentially be used as a new cell source for heart repair.

Ischemic cardiovascular disease is the leading cause of human death after 40 years of age. Heart disease eventually develops into heart failure, manifested by the loss of cardiomyocytes, combined with a significant reduction of myocardial contractility and heart function. Cardiovascular progenitor cells can differentiate into beating cardiomyocytes, blood vessel endothelial cells and vascular smooth muscle cells. All these cell types have great value in regenerative medicine.
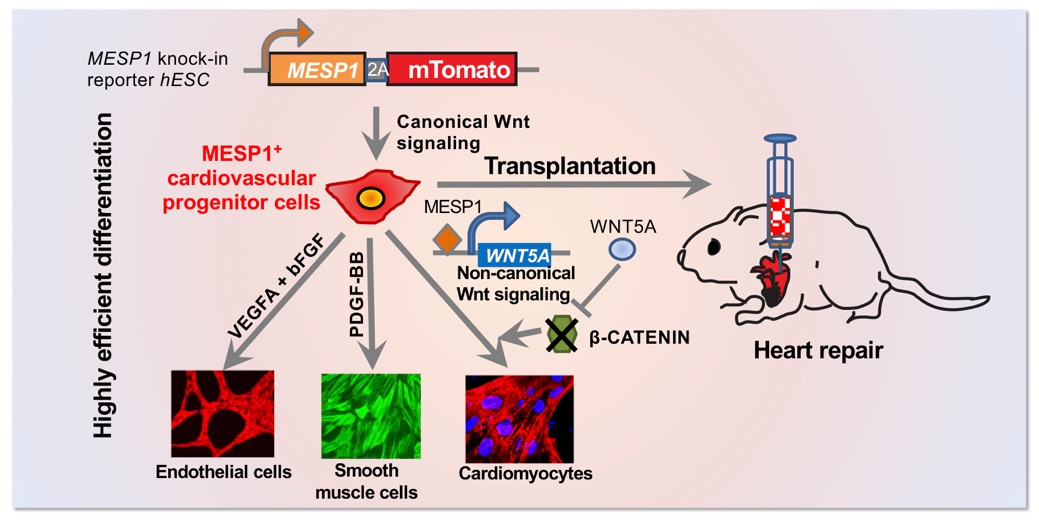
The researchers knocked in mTomato, a red fluorescent protein, at cardiac mesoderm marker gene MESP1 locus in human embryonic stem cells, to monitor the successful induction of cardiovascular progenitor cells. Using this MESP1-mTomato reporter cell line, the authors found that the expression of MESP1 requires the canonical Wnt signaling pathway. By controlling the canonical Wnt signal, the authors obtained nearly pure MESP1 cells which can efficiently differentiate into functional cardiac lineage cells in vitro, including cardiomyocytes (80%), smooth muscle cells (97%) and endothelial cells (30%). The authors also found that MESP1 can directly initiate the expression of WNT5A to inhibit the canonical Wnt signaling and promote cardiomyocyte differentiation. Finally, the authors showed that MESP1 cells could differentiate into endothelial cells and cardiomyocytes after being transplanted into the infarcted rat heart and improve the physiological function of the heart. This study provided a useful platform to study the early development of the human heart in vitro, supplied a method for efficiently obtaining MESP1 progenitor cells, and offered a potential new cell source for heart repair.
Dr Jie Na is the corresponding author of the paper, Drs Lin Wang and Fengzhi Zhang, from School of Medicine, Tsinghua University, are co-first authors. PhD students: Fuyu Duan, Rujin Huang, Xi Chen and research assistant Jia Ming, also contributed to this research. This work was supported by the funding from the National Key R&D Program of China, grant 2017YFA0102802, 2019YFA0110001. The National Natural Science Foundation of China (NSFC) Grant 31970819, 91740115, 31771108 and Tsinghua-Peking University Center for Life Sciences.
The web link of the article:
https://www.thno.org/v10p6898.htm
