Editor’s note
Early diagnosis can make a world of difference for cancer patients. This is particularly true for patients with lung cancer as the disease is usually diagnosed in a late stage when it might be already too late.
To improve the early diagnosis and treatment of lung cancer, Tsinghua’s Professor Peng Liu and his team have come up with a new drug sensitivity testing system that can produce results from organoids derived from lung cancer tumor tissue samples within a week, drastically shortening the time to diagnosis, which currently usually takes weeks or even months. Read on to find more about the testing system that could be a gamechanger in the fight against this chronic disease.
An organoid-based test to predict drug effectiveness returns individualized results within a week for the world’s deadliest cancer.
Hundreds of miniature organs, or ‘organoids’, grown in a nanoliter-scale microwell array system designed by Tsinghua researchers and their collaborators could soon be used to speed up decisions about personalized lung cancer drug treatment.
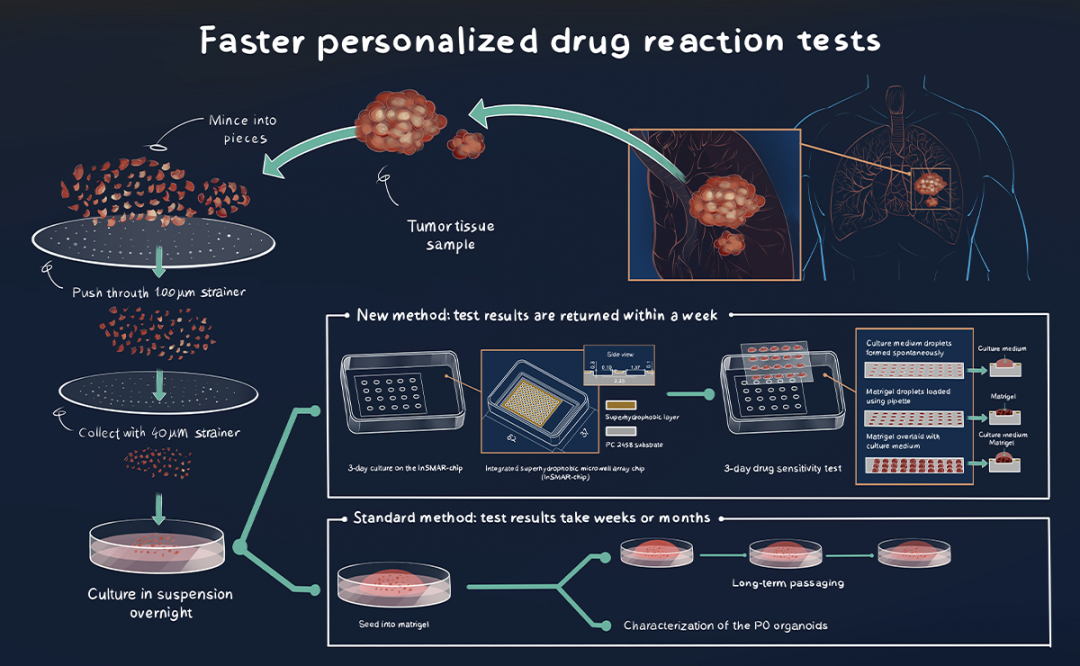
InNature Communicationsin May 2021, Tsinghua’s Peng Liu and his collaborators showcased how their new drug sensitivity testing system could produce results on lung cancer tumor tissue samples within a week.1
A long wait for personalized prediction
Patient-derived organoids (PDO) are useful for personalized drug testing because they reflect the basic structural and functional characteristics of the original cancer tissue. The three-dimensional cellular structures are grown from a patient’s biopsy or from surgically removed tissue samples.
Previous clinical studies on colorectal and gastroesophageal cancer suggest that tests with PDOs could be highly successful in predicting whether a given drug will be effective for certain patients.
Culturing enough PDOs to run a test to determine which drugs will be most effective usually takes weeks or even months. “The initial number of PDOs generated from lung cancer tissue ranges from several to thousands, but the number of PDOs required to complete drug screening in a typical well plate is in the order of millions,” explains Liu, a professor in Tsinghua’s Department of Biomedical Engineering.

Peng Liu and his team have come up with a new drug sensitivity testing system that can produce results from organoids derived from lung cancer tumor tissue samples within a week.
For lung cancer this is particularly problematic as the diagnosis is often late. The world’s deadliest cancer has broad symptoms, such as coughing and wheezing, and as a result, in the United States, more than two-thirds of lung and bronchial cancers cases between 2010 and 2016 were found at a late stage. “Patients are reluctant to wait until they receive drug sensitivity test results,” says Liu. “So shortened testing times will be significant to lung cancer patients in particular.”
In the May paper, the researchers were able to create a tiny and fast PDO production, isolation, and drug reaction testing system by processing lung cancer tumor tissues in a special integrated superhydrophobic microwell array called the ‘InSMAR-Chip’.
Reactions at the nanoscale
In creating a sample processing method that increased the number of organoids derived from patients, a key component was to culture the organoids at the nanoliter scale on the InSMAR-Chip, which contains 108 miniature-sized wells for measuring drug reactions.
Conventional cell culture techniques at microliter-scale volumes require prolongedin vitroexpansion to generate enough quantities of PDOs to take meaningful measures in that sized well. The nanoliter-scale wells on InSMAR-Chip make it possible to evaluate responses to anticancer drugs from just hundreds of organoids.

Xinzhao Sui assesses the clinical efficacy of drugs by comparing changes in lung cancer lesions before and after treatment.
The InSMAR-Chip builds on Liu’s previous development, the SMAR-Chip, and includes enhancements such as the use of new, water-repellent materials that help create uniform droplets in each of the wells.
Tests with the InSMAR-Chip can also flag acquired drug resistance; the team verified that lung cancer organoids derived from a patient with resistance to tyrosine kinase inhibitor therapy also showed greater resistance to the drug in the InSMAR-Chip test. “The drug responses reported using our method correlated strongly with genetic mutations and clinical outcomes,” concludes Liu.
Towards universal use
While the team conducted the current study on lung cancer, they have since applied the technology to colorectal, ovarian, liver, and pancreatic cancer.
But a challenge remains with the number of PDOs that can be generated from middle- and advanced-stage patients. “Many such samples are obtained through punctures, and the number of PDOs generated from these samples is very limited. The success rate of establishing organoids is low,” says Liu. “We will continue to optimize the sample processing method and organoid culture conditions, with the aim of making this technology more universal.”
